← All posts
Subtle Medical Receives FDA 510(k) Clearance and CE Mark Approval for SubtlePET™
MENLO PARK, Calif., Dec. 5, 2018 /PRNewswire/ — Subtle Medical, a privately-held medical device company focused on improving medical imaging efficiency and patient experience with innovative deep learning imaging technologies, announced today 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market SubtlePETTM. Subtle Medical also recently secured approval to affix the CE Mark on SubtlePET to begin marketing in the European Economic Area without restrictions.
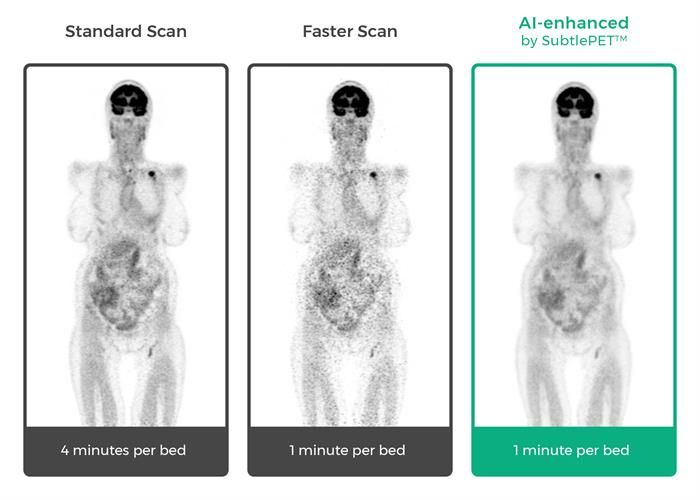
SubtlePET’s artificial intelligence (AI)-powered technology allows hospitals and imaging centers to enhance images from faster scans leading to an improved patient experience during imaging procedures, while boosting exam throughput and provider profitability. SubtlePET is currently in pilot clinical use in multiple university hospitals and imaging centers in the U.S. and abroad.
“Focusing Subtle Medical’s SubtlePET AI platform on faster image acquisition, we have been able to dramatically increase PET scan efficiency and provide a superior patient experience. SubtlePET technology allows us to scan a patient four times faster than normal, yet maintain equal image quality, not otherwise impacting work flow,” said Michael Brant-Zawadzki, MD, FACR, Hoag Hospital, Newport Beach, California. “This creates immediate ROI benefit for our hospital and a compelling value proposition. I’m looking forward to seeing more groundbreaking technology from the Subtle team.”
Subtle Medical’s AI solution enables completion of more exams in a day compared to conventional PET imaging without the need for capital expenditures. It reduces patient time in the scanner and helps hospitals and imaging centers enhance their bottom line in today’s competitive healthcare environment. The company’s technology utilizes deep learning algorithms that integrate seamlessly with any OEM scanner and PACS system to enhance images during acquisition without any interruption or alteration in the imaging specialists’ workflow. SubtlePET delivers a significant improvement in the image quality of noisy images resulting from shorter scans, which is particularly beneficial for children and those undergoing repeat PET exams.
SubtlePET is the first product in Subtle Medical’s growing portfolio of new AI technologies to receive FDA clearance. “This FDA clearance is a key milestone in Subtle Medical’s mission to bring novel and empathetic deep learning to improve patient satisfaction,” said Enhao Gong, PhD, Founder and CEO of Subtle Medical. “The accomplishment of having the first AI cleared for use in nuclear medicine applications validates our team’s strength and the commitment of our collaborators. Our focus on image acquisition and workflow differentiates us from other AI companies that are working on post-processing and computer-aided diagnosis products. We are not replacing radiologists–we are addressing the tremendous cost to U.S. healthcare by leveraging deep learning in imaging at the infrastructure level to enable better and higher quality care.”
Subtle Medical is developing additional products to be submitted for FDA clearance. A second product currently undergoing clinical evaluation is SubtleMRTM, which allows imaging centers to significantly accelerate MRI scans using the company’s AI solutions. SubtleGADTM is also being developed to reduce gadolinium dosage during imaging procedures.
In March 2018, Subtle Medical received the NVIDIA Inception Award for Top Healthcare+AI Startup Globally selected from over 3,000 AI contenders. The company was also selected as the first AI+healthcare startup for Bessemer Venture Partners’ Deep Health Seed Program. Most recently, it was named as a 2018 Minnies Award semi-finalist for Best New Radiology Vendor by AuntMinnie.com.


